All of us can remember the years in which Sri Lanka was very badly hit by dengue. At the same time all the media highlighted three words: Papaya leave juice. Some who got infected by Dengue used it without the approval of the MBBS doctors and while some did not use it. Anyway some got cured and some died. But up to date, no one knows who died and who survived and whether they took any papaya leave juice or not. Unfortunately a similar situation can be observed now in connection with COVID-19 and certain Ayurvedic medicines after almost half of a decade.
 By now it is obvious to all educated Sri Lankans that someone or an invisible supernatural force is blocking the easy and cheap ways of achieving good health for Sri Lankan people. This is done by using a very simple tactic. It is preventing any crucial Clinical Trials within Sri Lanka or stopping Sri Lanka from obtaining the assistance of developed foreign countries to get crucial Clinical Trials done. It is very easy to solve the issue if the Government can deploy intelligence officers and find out who is doing what in order to prevent Clinical Trials from taking place. On the other hand Sri Lanka can simplify any procedure according to local requirements and available resources just like how India does it.
By now it is obvious to all educated Sri Lankans that someone or an invisible supernatural force is blocking the easy and cheap ways of achieving good health for Sri Lankan people. This is done by using a very simple tactic. It is preventing any crucial Clinical Trials within Sri Lanka or stopping Sri Lanka from obtaining the assistance of developed foreign countries to get crucial Clinical Trials done. It is very easy to solve the issue if the Government can deploy intelligence officers and find out who is doing what in order to prevent Clinical Trials from taking place. On the other hand Sri Lanka can simplify any procedure according to local requirements and available resources just like how India does it.
On 14th July in 2017 (over three years ago) during a press briefing held in Colombo, (then) Clinical Head of the Centre for Clinical Management of Dengue and Dengue Hemorrhagic Fever, Negombo, Dr. Lakkumara Fernando said that the dengue patients should understand that a person or a substance (may be juice of papaya leaves) gets the credit when the dengue patient’s platelets count goes up naturally when the body responds to the virus naturally. Therefore regardless of whether a dengue patient takes a certain substance or not, his/her platelet count goes up naturally when the right time comes.
On October 16th in 2015 (over five years ago) during a press briefing held in Colombo, Dr. Hasitha Tissera, a Consultant Epidemiologist attached to the Epidemiology Unit of the Health, Nutrition and Indigenous Medicine Ministry said that giving green apple juice or papaya leaves juice to increase platelets should be taboo. One of the criteria which is used by doctors in identifying DHF early is a blood platelet count below 100,000. However, in the initial stages, if platelets are artificially increased by some means or another (eg; giving green apple juice or papaya juice) the clinician who is closely monitoring the fall of platelet count to diagnose the natural rise on recovery may be misled. This may affect patient management. Scientists have not yet discovered any compound having anti-leaking properties. Platelet reduction is just a feature of DHF and is not the cause of it. Therefore, if something is claimed to bring up platelets artificially it is not going to help DHF patients.
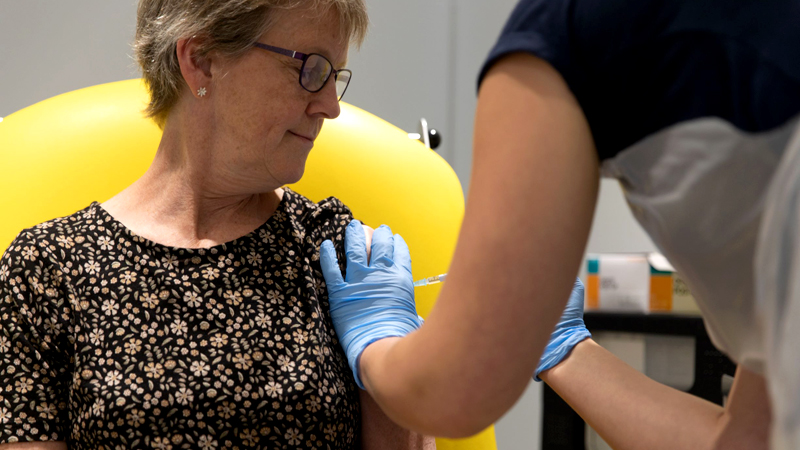
 Those are the views presented by medical experts at that time (several years ago) on using the juice of papaya leaves to increase the platelet count on dengue patients. Some of the experts said there are some other patients in state hospitals with very low platelet counts due to other disease (not dengue) and if Papaya leave juice is working, it should be able to increase their platelet count too. But nothing was done to find out anything more about it. Enabling Sri Lanka to conduct proper clinical trials with or without the assistance of foreign countries is the need of the hour. In fact, the Government has already announced that clinical trials will be conducted for COVID-19 vaccine candidates when they become available to Sri Lanka. Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioural research studies on human participants are designed to answer specific questions about biomedical or behavioural interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval for the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial. Their approval does not mean the therapy is ‘safe’ or effective, only that the trial may be conducted.
Those are the views presented by medical experts at that time (several years ago) on using the juice of papaya leaves to increase the platelet count on dengue patients. Some of the experts said there are some other patients in state hospitals with very low platelet counts due to other disease (not dengue) and if Papaya leave juice is working, it should be able to increase their platelet count too. But nothing was done to find out anything more about it. Enabling Sri Lanka to conduct proper clinical trials with or without the assistance of foreign countries is the need of the hour. In fact, the Government has already announced that clinical trials will be conducted for COVID-19 vaccine candidates when they become available to Sri Lanka. Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioural research studies on human participants are designed to answer specific questions about biomedical or behavioural interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval for the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial. Their approval does not mean the therapy is ‘safe’ or effective, only that the trial may be conducted.
pilot studies
Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary in size and cost, and they can involve a single research centre or multiple centres, in one country or in multiple countries. Clinical study design aims to ensure the scientific validity and reproducibility of the results. For example, Phase 3 Clinical Trials of the two major COVID-19 vaccines now in use had enrolled more than 30,000 participants each in several countries. Clinical trials for a number of other COVID -19 vaccines are still ongoing worldwide.
Certain functions necessary to the trial, such as monitoring and lab work, may be managed by an outsourced partner, such as a contract research organisation or a central laboratory. Only 10 percent of all drugs started in human clinical trials become approved drugs/treatments in any case.
Some clinical trials involve healthy subjects with no pre-existing medical conditions. Other clinical trials pertain to people with specific health conditions who are willing to try an experimental treatment. Minors are not usually recruited for clinical trials unless the particular treatment is targeted especially at that age group. Pilot experiments are conducted to gain insights for design of the clinical trial to follow.
There are two goals to testing medical treatments: to learn whether they work well enough, called "efficacy" or "effectiveness"; and to learn whether they are safe enough, called "safety". Neither is an absolute criterion; both safety and efficacy are evaluated relative to how the treatment is intended to be used, what other treatments are available, and the severity of the disease or condition. The benefits must outweigh the risks. For example, many drugs to treat cancer have severe side effects that would not be acceptable for an over-the-counter pain medication, yet the cancer drugs have been approved since they are used under a physician's care and are used for a life-threatening condition.
The sponsor designs the trial in coordination with a panel of expert clinical investigators, including what alternative or existing treatments to compare to the new drug and what type(s) of patients might benefit. If the sponsor cannot obtain enough test subjects at one location investigators at other locations are recruited to join the study.
During the trial, investigators recruit subjects with the predetermined characteristics, administer the treatment(s) and collect data on the subjects' health for a defined time period.
Data include measurements such as vital signs, concentration of the study drug in the blood or tissues, changes to symptoms, allergic reactions, and whether an improvement or worsening of the condition targeted by the study drug occurs. The researchers send the data to the trial sponsor, who then analyzes the pooled data using statistical tests. In most trials, two sets of volunteers are used, one gets the experimental drug or vaccine and the other, a placebo, which can be a non-drug pill or a saline solution (if a vaccine is being trialled). The participants do not usually know what they get.
Examples of clinical trial goals include assessing the safety and relative effectiveness of a medication or device:
- On a specific kind of patient
- At varying dosages
- For a new indication
- Evaluation for improved efficacy in treating a condition as compared to the standard therapy for that condition
lEvaluation of the study drug or device relative to two or more already approved/common interventions for that condition
While most clinical trials test one alternative to the novel intervention, some may expand to three or four and may include a placebo.
Except for small, single-location trials, the design and objectives are specified in a document called a clinical trial protocol. The protocol is the trial's "operating manual" and ensures all researchers perform the trial in the same way on similar subjects and that the data is comparable across all subjects. Moreover, those who conduct clinical trials have an obligation to protect the confidential personal and medical data of their subjects.
scientific method
As a trial is designed to test hypotheses and rigorously monitor and assess outcomes, it can be seen as an application of the scientific method, specifically the experimental step. The most common clinical trials evaluate new pharmaceutical products, medical devices, biologics, psychological therapies, or other interventions. Clinical trials may be required before a national regulatory authority approves general marketing of the innovation.
Similarly to drugs, medical or surgical procedures may be subjected to clinical trials, such as case-controlled studies for surgical interventions.
As we write this, clinical trials are underway for a COVID-19 monoclonal antibody treatment, a HIV vaccine, a breast cancer vaccine, dengue and malaria vaccines and a host of other diseases.